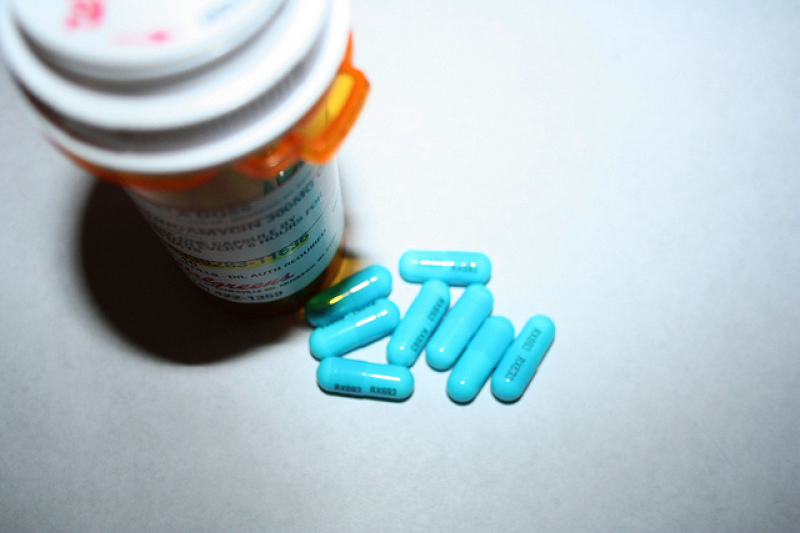
The Food and Drug Administration announced that they will implement changes on labeling prescription drugs to help pregnant women determine medicines that are safe for consumption easier than before.
The FDA said that the old system that uses letters A, B, C, D and X to categorize drugs will be improved by implementing three detailed subsections to give pregnant information on the risks and benefits of prescription drugs that they are planning to take.
"The letter category system was overly simplistic and was misinterpreted as a grading system, which gave an over-simplified view of the product risk," Dr. Sandra Kweder, an official from FDA's Center of Drug Evaluation and Research, said in a statement. "The new labeling rule provides for explanations, based on available information, about the potential benefits and risks for the mother, the fetus and the breastfeeding child."
Under the new rule, drug manufacturers should use three subsections in labeling - "Pregnancy," "Lactation" and "Female and Males of Reproductive Potential" - to provide detailed information about the risk of using the drug during pregnancy and breastfeeding.
The "Pregnancy" subsection will inform consumers about proper dosing and possible side effects of the drug to developing fetus. If there is potential risk to pregnant women, manufactures should also include information about existence of pregnancy registries that study effects of drugs or biological products during pregnancy.
The "Lactation" subsection provides details about the estimated amount of drug that could go in breast milk and the potential effects to a breastfed child, while the "Female and Males of Reproductive Potential" subsection gives information about pregnancy testing, contraception and infertility.
"This is a much needed change and will provide patients and health care providers will specific and relevant information, including data from drug trials and registries," Dr. Joanne Stone, director of Maternal Fetal Medicine at The Mount Sinai Hospital, said in an interview with CBS News.
The new rule, which will take in effect in June, will be beneficial to many women in the United States. According to FDA, there are more than six million pregnancies in the country per year, and most pregnant women, who have history of asthma and high blood pressure, take at least three prescription drugs during pregnancy.